Aim: A registered study to investigate the efficacy and safety of bendamustine plus melphalan conditioning for autologous stem cell transplantation (ASCT) in multiple myeloma (MM) (ChiCTR2100047782).
Method: Bendamustine and melphalan were given to patients at the dose of 150 mg/m 2 on Days -5 and -4 and 70 mg/m 2 on Days -3 and -2, respectively. Progression-free survival (PFS) and overall survival (OS) were used to assess the efficacy and adverse events were used to assess the safety of the conditioning regimen.
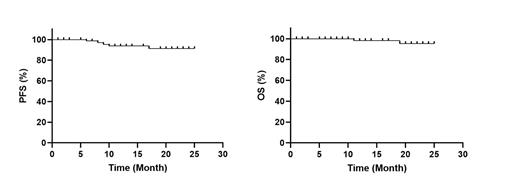
Results: A total of 80 patients were included in our study. Of them, 45 patients achieved complete response (CR); 7 patients achieved very good partial response (VGPR), and 28 patients achieved partial response (PR) before ASCT. With a median follow-up period of 16 months, the overall PFS rate was 91%, and the overall OS rate was 95%. Patients with CR pre-transplantation were all in CR until the last follow-up; two patients with VGPR pre-transplantation relapsed and one of them died; four patients with PR pre-transplantation relapsed and one of them died. The median time to neutrophile and platelet engraftment were 11 (9-13) and 13 (10-18) days. Three patients experienced sepsis during ASCT. Vomiting occurred in 45 (57.2%) patients; diarrhea occurred in 27 (34.2%) patients, transaminase elevation was observed in 18 (22.3%) patients; mucositis was found in 16 (18.8%) patients. All these adverse events were grade 1-2, and no grade 3-4 adverse events were observed.
Conclusion: Bendamustine plus melphalan regimen is promising for ASCT in MM patients, and the long-term efficacy and safety remain to be investigated.
Disclosures
No relevant conflicts of interest to declare.